Overcome the challenges of PMS2 variant calling with Devyser LynchFAP – our new NGS solution
Devyser LynchFAP is the latest addition to Devyser’s expanding hereditary cancer screening...
Genetic diagnostic testing for cancer is centerstage as oncology transitions to precision medicine and personalized treatment. Our fast, accurate DNA tests assist in detection and prevention – and help drive the development of individualized therapies. At a time when genetic oncology grows at unprecedented speed, we believe that, in some cases, less is more.
Cancer treatment is changing fast. The shift to precision medicine and personalized therapies is rapid and real. Genetic testing is a crucial enabler of this transformation..
At a time when genetic oncology is growing at unprecedented speed, we believe that less is sometimes more.
Increased preventive DNA cancer screening makes it possible to identify more individuals at risk. It can also help combat the rising incidence of some cancer types in an aging population.
Our diagnostic tests for breast, ovarian and other cancers identify clinically significant variants with unsurpassed accuracy. Their high user convenience and fast, actionable results support the drive towards individualized cancer diagnostics.
A single-tube format generates a simple workflow with short hands-on time. User-friendly analysis software ensures effective automation throughout the process.
The end-to-end procedure is fully CE IVD-certified in compliance with the EU’s In-Vitro Diagnostic Medical Device Directive, guaranteeing robust results in clinical use that you can trust.

One tube per patient sample - no sample splitting
45
Less than 45 minutes hands-on time
CE-IVD
CE-IVD certified solution – from sample to result
Breast cancer is the leading cause of cancer-related death in women. An estimated one in eight will develop the disease in their lifetime, while ovarian cancer is responsible for more deaths than any other cancer of the female reproductive system. We provide diagnostic solutions to cover women along the whole way – from prevention to treatment. Our germline mutation analysis identifies those who are at risk of developing breast and ovarian cancer and detects individuals who may benefit from risk-reducing strategies. Our somatic mutation analysis identifies women who may benefit from targeted treatment using PARP inhibitor.

News | June 26, 2025
Devyser launches Devyser HLA Loss: A high-precision NGS assay for post-transplant researchNews | October 29, 2024
Devyser seeks to secure FDA approval for NGS test for kidney transplant monitoringNews | October 28, 2024
Devyser Compact achieves Class III approval in China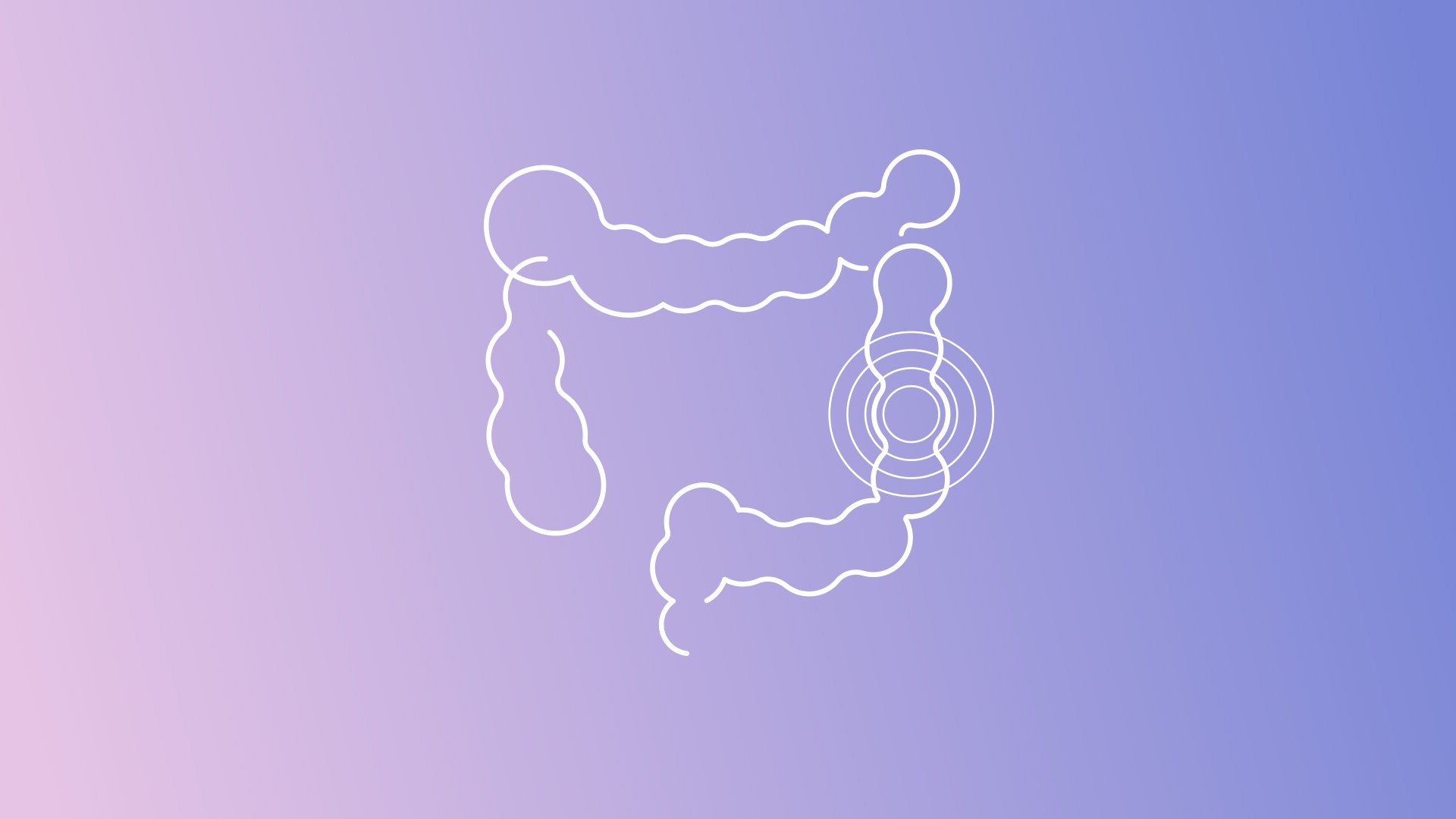
Devyser LynchFAP is the latest addition to Devyser’s expanding hereditary cancer screening...
Read More
-2.png)
Devyser is thrilled to announce the launch of two new products, Devyser LynchFAP and Devyser BRCA...
Read More

As a lab manager, you know the importance of streamlining workflows to maximize productivity and...
Read More

Do you know a man who has participated at least once in “Movember”? Every year, thousands of men...
Read More